Wednesday, October 1, 2014
Determination of an Activity Series
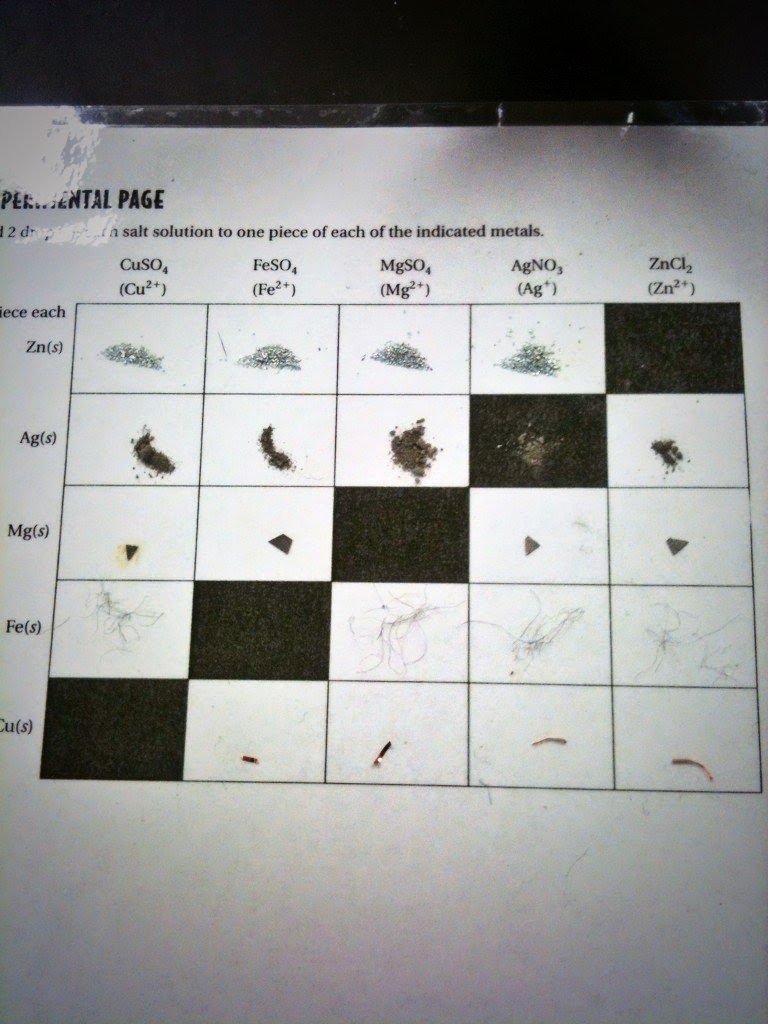
All metals are not equally active. This is because not every metal will loose electrons as easily as another will. A single replacement reaction is when an element is replaced by another in a compound. A redox reaction is a reaction that involves the transfer of electrons between two substances. A redox reaction can have different types of reactions. A single replacement reaction as a redox reaction is a reaction that replaces an element in the reactants with another element in the products. Here are the rankings of the metals from most reactant to least reactant, and their reduction equations.
Subscribe to:
Post Comments (Atom)

No comments:
Post a Comment