- This weekend I have started my poster for my lab experiment and I have started studying for the ACT.
- I have learned how to do redox reactions properly! Finally.
- I plan on practicing my reaction types and determining their products and studying more for my ACT.
Tuesday, October 14, 2014
3 Questions
Wednesday, October 1, 2014
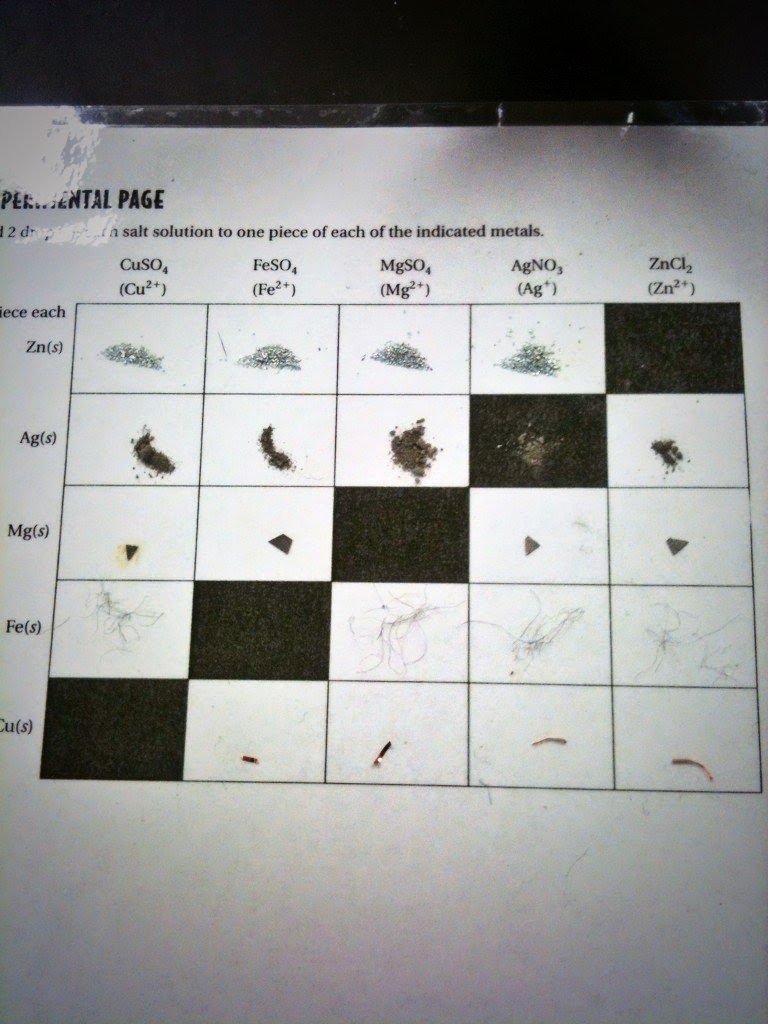
Determination of an Activity Series
All metals are not equally active. This is because not every metal will loose electrons as easily as another will. A single replacement reaction is when an element is replaced by another in a compound. A redox reaction is a reaction that involves the transfer of electrons between two substances. A redox reaction can have different types of reactions. A single replacement reaction as a redox reaction is a reaction that replaces an element in the reactants with another element in the products. Here are the rankings of the metals from most reactant to least reactant, and their reduction equations.
Subscribe to:
Posts (Atom)