1. Recently I have completed highschool. As in today. I am finally done.
2. Recently I have learned that I don't have to see alot of the people from highschool around college because we won't be in a tight knit school. And for that I am glad. Its about time my life becomes drama free.
3. What I need to work on is my self esteem. I think being in a class with all of those genius people made me think that I wasn't smart enough. I need to work on that.
Friday, May 8, 2015
Thursday, May 7, 2015
Reaction Rates
The reaction coordinate can be used to find whether a reaction will continue and how the potential energy of a system changes. The reaction coordinate is a parametric curve that shows the activation energy of a reaction down to the energy of the products produced. Things that affect the potential energy of a particle are its mass, because smaller particles tend to move faster and produce more collisions, and pressure. An exothermic reaction, where bonds are being broken and then newly formed, the potential energy goes from a higher state to a lower state over the course of the reaction. The opposite occurs in an endothermic reaction. This reaction coordinate can tell us how quickly or slowly a reaction will occur, or if it doesn't occur at all. It all depends on the total energy of the reaction (potential plus kinetic). If the total energy is low, the reaction will have a slower rate. If the total energy is high, the reaction will have a faster rate.

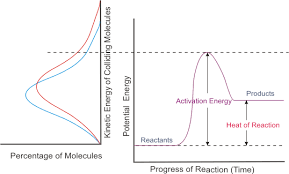

Saturday, April 25, 2015
3 questions
1. Recently I learned how to correctly find the pH of a substance and then find the molarity of it.
2. Recently I completed my practice AP test and I need to improve my scores but I at least got a 3 so that's helpful.
3. I need to work on finding x in the Rice equation.
2. Recently I completed my practice AP test and I need to improve my scores but I at least got a 3 so that's helpful.
3. I need to work on finding x in the Rice equation.
Wednesday, April 15, 2015
Calorimeter explore
The difference between an acid and a base in the terms of pH is that acids have more hydrogen ions and bases have more hydroxide ions. The link between pH and pOH is the sum of both equal 14. pH is related to H+ ions because it measures the concentration of H+ ions with 1 being the most acidic to 14 being the most basic. pOH is the measure of the concentration of OH- ions. The universal indicator reacted with every buffer, unlike the other indicators that had limited range of reactions. For a neutralization of 5 or 9, I would choose different indicators considering they are on opposite sides of the spectrum. Indicators are substances that react with either the H+ or OH- ions in another substance, showing a change in color when they react and giving evidence of neutralization. The universal indicator can react with almost any substance, the other indicators have a smaller range of reactivity.





Tuesday, April 14, 2015
3 Questions
1. Recently I've completed my graduation announcement cards. It's coming up so fast!
2. Recently I've learned about letting go of things that aren't in my power. Kind of a hard lesson to grasp.
3. I've been having trouble with some of the math for equilibrium equations, so I'm going to practice those enough that I can do them whilst sleeping.
2. Recently I've learned about letting go of things that aren't in my power. Kind of a hard lesson to grasp.
3. I've been having trouble with some of the math for equilibrium equations, so I'm going to practice those enough that I can do them whilst sleeping.
3 questions
1. Recently I've learned (completely learned) how to finally find molarity after being so confused about it most of the time. It's the small things.
2. Recently I've completed my spring cleaning so that's always refreshing.
3. Right now I'm struggling to find motivation to do anything other than sleep or eat, so I need to work on that. I think I'm just going to try to push myself and finish better than I started.
2. Recently I've completed my spring cleaning so that's always refreshing.
3. Right now I'm struggling to find motivation to do anything other than sleep or eat, so I need to work on that. I think I'm just going to try to push myself and finish better than I started.
Friday, April 10, 2015
Nitrogen Dioxide and Dinitrogen Tetraoxide
The two substances in this reaction are NO2 and N2O4. This reaction is a reversible reaction, which is how it reaches equilibrium.takes more energy. These two are the main components in smog, and the formation of N2O4 reaction The whole reaction is 2NO2 --> N2O4 with an enthalpy of -58.0 kJ/mol of N2O4
The reaction is temperature favored. The cold ice water favors the N2O4, therefore changing the rate of the production of N2O4 and boosting the molarity of it. The heat from the bunsen burner favors the NO2 side of the reaction, making the rate of NO2 faster than the rate of N2O4.
You can tell the cold favors the N2O4 because N2O4 is naturally a clear gas. But as soon as you move the test tube into the boiling water the gas turns a yellowish brown color, showing that the reaction has reversed and the NO2 is more favored because NO2 in gas form is that color. the NO2, when placed in the boiling water, pops. This is due to the greater number in moles. Le Chatelier's principle can be used to predict the effect of the change of conditions in an equilibrium reaction. The equilibrium shift has to do with enthalpy of the reaction. One side if the reaction favors the release of energy, and one favors the absorption if energy. The color change has to do with the equilibrium trying the compensate for the change that the rate of the reaction is kept the same. The reaction at room temperature is a equal mixture of both NO2 and N2O4 color with neither one favored more than the other, compared to the favoring of the reaction side depending on the temperature.
Friday, March 13, 2015
3 Questions
1. Recently I have completed unit 7. We tested on it and I didn't do as bad as I thought I would.
2. Recently I have learned about the order of reactions. I thought I wouldn't understand this unit because of the math but it's actually not hard.
3. I have been struggling with motivation lately. I plan on just sucking it up and kicking it into high gear.
2. Recently I have learned about the order of reactions. I thought I wouldn't understand this unit because of the math but it's actually not hard.
3. I have been struggling with motivation lately. I plan on just sucking it up and kicking it into high gear.
Thursday, March 5, 2015
VSEPR Lab
There are four types of solids: Covalent-network which are joined by an extensive network of covalent bonds, molecular which discrete molecules that are linked together by van der Waals forces, ionic which are cations and anions attracted to each other, and metallic which share a network of highly delocalized electrons. We used melting points, solubility, and electrical resistance as the defining properties. I would predict that citric acid was an ionic solid, zinc was metallic, charcoal was molecular, CaCl2 was covalent network, and sand was also metallic. Citric acid is a molecular solid, Zinc is a metallic solid, charcoal is a covalent-network, CaCl2 is ionic, and sand is also molecular.
Saturday, February 28, 2015
3 questions
1. Recently I have completed my unit 7 test.
2. I have just now gotten the hang of hybridization.
3. Still struggling with some basics from former units, I plan on studying for the AP test.
2. I have just now gotten the hang of hybridization.
3. Still struggling with some basics from former units, I plan on studying for the AP test.
Friday, February 13, 2015
3 Questions
- I have recently completed the last unit on electron configuration. I must say its been my favorite unit besides electrochemistry.
- Recently I have learned how to do a proper Lewis Structure. Before I was just giving all the elements 8 electrons, now I know the rules and the number of bonds one can make.
- I have some trouble remembering that in order to count the number of electrons in a molecule you have to add the number of the column. I keep thinking for some reason I'm supposed to put down a number that corresponds to their ions. So I just have to keep in mind that its the number of the column.
Tuesday, February 10, 2015
Soap & Pepper
The different intermolecular forces are hydrogen bonding, London-dispersion forces, and dipole-dipole.
Water's intermolecular forces experience a dipole because of the slightly larger charge on one side than the other.
Water's intermolecular forces experience a dipole because of the slightly larger charge on one side than the other.
Soap seems to have polar (hydrophilic) and nonpolar (hydrophobic) properties towards water because it caused the pepper to spread back but then it dissolved into the water. The intermolecular forces it experiences are London-dispersion forces. When the soap was added to the pepper water, as soon as the soap hit the water the pepper spread away from it as if they were repulsing each other. Then after awhile the soap dissolved completely into the water. This happened because the soap held nonpolar qualities and pushed away the polar pepper sitting on top of the polar water. However, the soap also possessed polar qualities too, because it was aqueous with the water and dissolved fast.
Friday, January 30, 2015
3 questions
- Recently I have completed 3 books in two days. I have no life.
- I have learned a little more about spectroscopy and how to use a spectrophotometer, which I must say is pretty nifty.
- I am struggling with calculations, mostly because I can't seem to grasp the basics. I need to work on those by just going over the stoich and measurement rules.
Wednesday, January 28, 2015
Coulumb's Law Exploration
- Coulumb's Law is a law describing the electrostatic attraction between two charged particles. The equation is E (energy) is equivalent to Q1 + Q2 over d (distance). The Q's are the different charges.
- Distance and charge both effect the energy in a different way. Distance is indirectly related to energy, the greater the distance, the less the energy to remove an electron from the atom. Charge and energy are directly related, so the greater the charge the greater the energy required to remove an electron from the atom.
- Removing an electron is an endothermic process because it requires energy to be absorbed by the electron for it to overcome the attractive forces of the nucleus. Its not exothermic because it isn't letting go of energy. If it was, the electron would be moving closer to the nucleus, not away from it.
- The amount of energy required to remove an electron is based on the distance from the electron and the charge of the nucleus, and also the net charge the nucleus has on the electron. The further from the nucleus, the less energy it takes to remove and electron. The closer to the nucleus, the harder it is to remove. The valence electron is the furthest electron from the nucleus, and the net charge on the valence electron is usually less than the net charge on the electron that are in closer orbitals.
- The energy to remove an electron compared the energy to excite and electron are much different. If the electron is in a higher orbital, the energy to remove it is low. However, the energy to move an electron from a low orbital to a high orbital, or from ground state to an excited state, needs a lot of energy because its overcoming the attractive electrostatic forces. Now if the electron that you're removing is closer to the nucleus, it could potentially take around the same amount of energy to remove it as it would to move that electron to an excited state.
Friday, January 16, 2015
3 Questions
- I have completed Allstate recently. It went really well, we even got an encore. That was definitely the best allstate year yet.
- I am now extending my knowledge on light energy and electron transmission. I have to say, besides electrochemistry this is my favorite unit so far.
- I am still struggling with the thermodynamic unit, but hopefully with the help of unit 6 I will start putting two and two together.
Explore blog
The relationship between energy, wavelength and frequency of light is indirectly related. The higher the wavelength range, the lower the frequency and the lower the energy. This description fits with the color red. The wavelength is long, with a lot of space in between the crests and troughs. The photon energy is low, it ranges from 269 to 318 Joules. Light emission demonstrates electron transition by releasing the color the energy change corresponds to. If an electron moves from a high energy state to a lower energy state, it will likely give off a higher frequency color, like violet or blue, given the amount of energy it is releasing to move that electron closer to the nucleus. Say an electron moved from energy level 6 to energy level 2. Since it would lose alot of energy because it moved much closer to the nucleus, it would give off a high frequency with a high photon energy, such as violet, which has a short wavelength of 380 to 440 nm. Say it was the other way around, and the electron was moving from a lower, or ground, state, to a higher, or excited, state. The electron would absorb the color light corresponding to its wavelength because the electron has to gain energy to overcome the forces of attraction with the protons in the nucleus. Light can be used to measure the electron transition of electrons simply by observing the color given off and relating it to its wavelength, frequency and energy.
sources: http://en.wikipedia.org/wiki/Light
Subscribe to:
Posts (Atom)




