1. Recently I have completed highschool. As in today. I am finally done.
2. Recently I have learned that I don't have to see alot of the people from highschool around college because we won't be in a tight knit school. And for that I am glad. Its about time my life becomes drama free.
3. What I need to work on is my self esteem. I think being in a class with all of those genius people made me think that I wasn't smart enough. I need to work on that.
Friday, May 8, 2015
Thursday, May 7, 2015
Reaction Rates
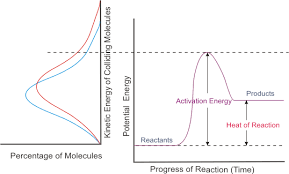
The reaction coordinate can be used to find whether a reaction will continue and how the potential energy of a system changes. The reaction coordinate is a parametric curve that shows the activation energy of a reaction down to the energy of the products produced. Things that affect the potential energy of a particle are its mass, because smaller particles tend to move faster and produce more collisions, and pressure. An exothermic reaction, where bonds are being broken and then newly formed, the potential energy goes from a higher state to a lower state over the course of the reaction. The opposite occurs in an endothermic reaction. This reaction coordinate can tell us how quickly or slowly a reaction will occur, or if it doesn't occur at all. It all depends on the total energy of the reaction (potential plus kinetic). If the total energy is low, the reaction will have a slower rate. If the total energy is high, the reaction will have a faster rate.

Subscribe to:
Comments (Atom)