- Recently I have completed yet another ACT. I think I did better than my last one.
- I have recently learned how to make a long lasting fire for my fireplace in my bunkhouse, and also how to fix a heater. (My heater decided to cease to work)
- I plan on spending my Christmas break learning allstate music and working and relaxing.
Thursday, December 18, 2014
3 Questions
Friday, December 5, 2014
3 Questions
The biggest task I've completed recently is getting a 24 on my first ACT. I thought it was a pretty good beginning score but I'm still aiming for higher.
Something I have learned recently is not stressing over everything. I have been stressing a lot over school and life and its too much energy to stress.
I plan on taking the SAT this weekend and my 2nd ACT next weekend and then finishing this year off strong.
Something I have learned recently is not stressing over everything. I have been stressing a lot over school and life and its too much energy to stress.
I plan on taking the SAT this weekend and my 2nd ACT next weekend and then finishing this year off strong.
Saturday, November 22, 2014
Sticky situations explore
Solvation is also called dissolution, and its the process of attraction between the molecules of a solvent and the molecules of a solute. Solubility is the amount of solute that can dissolve or dissociate a solvent. The process of chromatography is the figuring out the polarity of a substance by seeing how well it sticks to paper. Intermolecular forces are defined as the attraction of forces that molecules from different compounds feel based on their dipole moments (the polarity of the molecules). Intermolecular forces affect solvation because if the molecules have strong intermolecular forces, they don't solvate as easily as molecules with weak intermolecular forces. Cohesion forces are intermolecular forces that tend to resist breaking apart. A good example of cohesion forces would be hydrogen bonding because hydrogen bonding is a strong intermolecular force. Adhesion forces are intramolecular forces, which is the attraction between two different molecules. The term "like dissolves like" is employed to show that if a substance is polar, it is more likely to solvate in a polar solvent than a nonpolar solvent, and vice versa. This explains chromatography results by showing that the ink or dye will stick to the paper very effectively if it has the same polarity as the paper. If the dye doesn't have the same polarity, it will not be as cohesive.
Friday, November 21, 2014
3 questions
This past week I've been catching up in my classes. I am getting better at scheduling and writing stuff down in my calendar. Also I applied for a vocal scholarship for Eastern New Mexico and got invited to a dinner to learn about New Mexico Tech. I plan on improving my grades as finishing this semester strong and also eating alot on thanksgiving break.
Monday, November 17, 2014
Electrolysis Lab (again)
At the anode, the reaction is 2H2O(l) --> O2(g) + 4e- + 4H+ which is the sight of oxidation. The cathode's half reaction, 2H2O(l) + 2e- ==> 2H2(g) + 2OH- , is the sight of reduction. The difference between the galvanic, or voltaic, cell and the electrolytic cell is the spontaneity. Galvanic cells are spontaneous, whereas electrolytic cells are not. Electrolytic cells require an outside energy in order to react, which means this non-spontaneous cell has to be hooked up to something that will give it voltage so there is an actual redox reaction. Galvanic cells can react on there own by being placed in their own aqueous solution and completed with a wire and a salt bridge to keep the solutions neutral.
Calculations: For the amount of oxygen gas I got .00065 moles of O2 gas
For the amount of hydrogen gas I got .0026 moles of H2 gas

Calculations: For the amount of oxygen gas I got .00065 moles of O2 gas
For the amount of hydrogen gas I got .0026 moles of H2 gas
3 questions #7
This new section is all about gas pressure and volume and temperature. Its alittle hard to grasp but I am slowing understanding. I learned which variables are direct and indirect relationship-wise. I also learned alot of new laws and measurements that I never quite learned before this class. I plan on adding onto how much I know about measurements and memorizing the different laws.
3 questions # 6
- I have learned a lot about how batteries work, I did really well in the last section all about electrochemistry. It made sense to me which was quite surprising. I got a 75 on the test a a perfect score on the quiz, and I am very proud. I plan on applying my newfound understanding to the next section.
Tuesday, October 14, 2014
3 Questions
- This weekend I have started my poster for my lab experiment and I have started studying for the ACT.
- I have learned how to do redox reactions properly! Finally.
- I plan on practicing my reaction types and determining their products and studying more for my ACT.
Wednesday, October 1, 2014
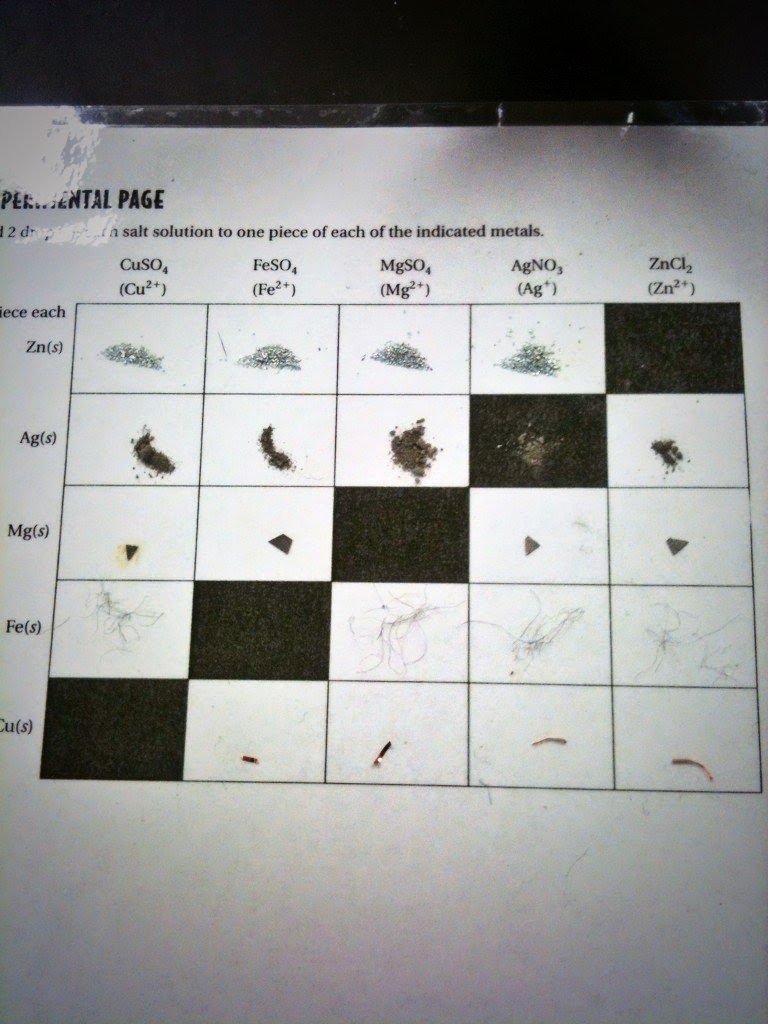
Determination of an Activity Series
All metals are not equally active. This is because not every metal will loose electrons as easily as another will. A single replacement reaction is when an element is replaced by another in a compound. A redox reaction is a reaction that involves the transfer of electrons between two substances. A redox reaction can have different types of reactions. A single replacement reaction as a redox reaction is a reaction that replaces an element in the reactants with another element in the products. Here are the rankings of the metals from most reactant to least reactant, and their reduction equations.
Thursday, September 25, 2014
Acid/Base Titration Lab
A Bronsted-Lowery acid is any substance that donates a Hydrogen atom, and a Bronsted-Lowery base is substances that accept a Hydrogen atom. All acid base equations have the hydrogen in common whether it is a cation or an anion. Titration is the process of finding the unknown molarity of one solution by slowly adding a solution of a known concentration to the unknown.
Small Scale Reactions
In this experiment we tested to see what anions and cations would precipitate when reacted. Then we found the net ionic equations for each reaction, if there was one. Solubility rules played a large part in the experiment. We learned what was soluble in water, and what wasn't. That's why some of the solutions precipitated when reacted, because the solutions created a solid when they reacted. The molecular, ionic, and net ionic formulas can all be found with each other unless you have only the net ionic equation. Here is our experiment sheet for the precipitate reactions.
Friday, September 12, 2014
3rd 3 questions
- I am understanding the stoich more and more every time we use it, also I understand molarity and molality, and the solutions parts (solute and solvent).
- I need a bit of work on finding the charges and utilizing them in the net ion equations, but I am getting the hang of finding the charges.
- I plan on taking notes on charges and doing a few extra steps while finding the net ion equations.
Wednesday, September 10, 2014
Small Scale Reactions
Using these small scale reactions with the baking soda and carbon dioxide, I can graphically prove the conservation of mass, the stoichiometric relationships, and the limiting reagents.
Water and Electrolytes
A solution is a mixture of a solute and a solvent. An electrolyte is an ionic compound composed of anions and cations that conduct electricity. Here is a particle diagram of the salt solution.
I created two different concentrations of the salt solutions. Here are the qualitative demonstrations of this difference.
My teacher's results My results
Beaker 3
Beaker 1
Beaker 2
You can mathematically show the difference in concentration by measuring the amount of electrolytes in each beaker. You can tell which beaker is D.I. water, tap water, or salt water by measuring the amount of electrolytes by putting in the copper wires to see whether it conducts electricity or not. I took advantage of the appearance of the solution and the way it conducted (or didn't conduct) electricity to find out which one was which. I recreated my teacher's results by using D.I. water for no light, tap water for dim light, and D.I. water with a few grams of salt added for bright light. I had an error in my calculations; I had used tap water plus salt at the beginning for beaker 2, but I ended up having D.I. water plus salt for the final trial. The final concentrations were 700ml of D.I. water for beaker 3, 600ml tap water for beaker 1, and 760ml of D.I. water combined with 3.16g of salt for beaker 2. My results came very close compared to my teachers, as shown above.
Saturday, August 30, 2014
2nd Three Questions
- The tasks I have completed are how to find empirical formulas, molecular formulas, and how to do stoich properly.
- What I can understand is the stoich conversions, and empirical/molecular formulas. I am still alittle fuzzy on the metric conversions.
- What I plan to do is to study the metric conversions so I know them right off the top of my head, that way stoich will come a lot easier to me.
Tuesday, August 26, 2014
Electrolyzing Water Lab
The qualitative evidence to support the balanced chemical reaction is the fact that you could visually observe both gases and which was which gas by the amount in the tubes, and the pH indicator showed the difference. You could collect quantitative data to prove the balanced reaction by justifying whether the reaction gained electrons or lost electrons. The reduction and oxidation equations show that.
Particle Diagram
Tuesday, August 19, 2014
Hydrate Explore Lab
A mixture is different from a pure substance because a pure substance is only one type of atom/molecules, whereas a mixture is a number of different pure substances mixed together. The Hydrate lab uses the technique heat to extract water molecules from the hydrate. Because all that is taken away from the hydrate is the water molecules, we can conclude that hydrate is a pure substance. You can support your conclusion mathematically by finding the percent composition. To find the percent composition, you need to divide the difference between the hydrated hydrate and the dehydrated hydrate, divided by the difference of the evaporating dish and hydrate, then multiply by 100. To evaluate the validity of your results, first you need to divide the part by the whole, then multiply it by 100. That number is going to be the ideal percent composition. Then you use stoichiometry to evaluate how close your results are.
Exothermic reaction in the Hydrate
Percent composition equation
Stoichiometry equation
Saturday, August 16, 2014
Three Questions
- The tasks I've completed are the chromatography lab and the online homework and the atomic mass online worksheet.
- I am starting to understand relative atomic mass and the inter/intramolecular forces, and I'm getting back the hang of nomenclature. I think what I could work on is calculating atomic mass and figuring out atomic isotopes, and I could use a bit of practice on polyatomic ions.
- What I am going to do to understand or remember what I don't get or forgot is to look back on my old notes from Chemistry and study my polyatomic ions.
Wednesday, August 13, 2014
Chromatography Lab
This experiment is dealing with the separation of dyes using Chromatography. The multiple trials done with the dyes and the different solvents used conclude that this experiment follows the scientific method. The main solvent, the paper, is what the dye, or solute, is attracted to. The other solvents (NaCl, Alcohol, and the Chromatography solution) are what help the dye travel up the paper. If the dye is more attracted to the paper, it will stay either in the same place or barely move with the solvent up the paper. If the liquid solvents are more attractive to the dyes, the dye will travel with the solvent up the paper. Whether the dye moves up the paper depends on the polarity of the solvent. Also, since the dye is a homogeneous mixture, the dye will separate into its original forms depending on how polar the solvent is. The chemical composition is different in all solvents, making the dye move up the paper differently. All solvents have different molecular structures, therefore their attraction towards the dyes differentiate. With the NaCl solution, the dyes moved further up the paper and are separated into its separate colors. With the alcohol, the dyes moved together and didn't fade or spread as much as the NaCl solution. With the FDC solution, the dye didn't move at all. The FDC dyes were supposed to be the control of this experiment, however they did not work according to plan. The qualitative evidence is that the dyes were proven to be a mixture according to the chromatography paper. The separate dyes used to make a new mixture was separated when in contact with certain solvents.
Example:
The quantitative data is the distance of the component compared to the distance of the solvent line/mobile phase. The different dyes in the mixtures were obviously set apart when the solvent traveled up the paper.
Examples:
Data for Rf
Vis-a-vis: NaCl
.8cm on the 10cm mark
original color - blue
Data for Rf
Crazart: Alcohol
.8cm on the 3cm mark
This is the particle diagram explaining dye travelling
The 3cm mark's dye has a strong attraction to the solvent because of the molecular structure and the polarity. The 2cm mark's dye is not as attracted to the solvent, and the 1cm mark's dye is not attracted to the solvent at all. This lab reinforced the idea of intramolecular and intermolecular forces, which one is chemical and which one is physical, and the idea of 'like dissolves like' due to the polarity and nonpolarity of a substance. It also established the difference between physical and chemical properties and changes by using a certain separation technique.
Subscribe to:
Comments (Atom)













.jpeg)
.jpeg)
.jpeg)







