1. Recently I have completed highschool. As in today. I am finally done.
2. Recently I have learned that I don't have to see alot of the people from highschool around college because we won't be in a tight knit school. And for that I am glad. Its about time my life becomes drama free.
3. What I need to work on is my self esteem. I think being in a class with all of those genius people made me think that I wasn't smart enough. I need to work on that.
Becca AP Chem
Friday, May 8, 2015
Thursday, May 7, 2015
Reaction Rates
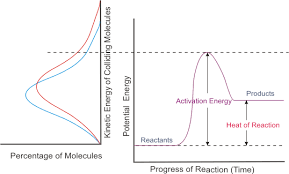
The reaction coordinate can be used to find whether a reaction will continue and how the potential energy of a system changes. The reaction coordinate is a parametric curve that shows the activation energy of a reaction down to the energy of the products produced. Things that affect the potential energy of a particle are its mass, because smaller particles tend to move faster and produce more collisions, and pressure. An exothermic reaction, where bonds are being broken and then newly formed, the potential energy goes from a higher state to a lower state over the course of the reaction. The opposite occurs in an endothermic reaction. This reaction coordinate can tell us how quickly or slowly a reaction will occur, or if it doesn't occur at all. It all depends on the total energy of the reaction (potential plus kinetic). If the total energy is low, the reaction will have a slower rate. If the total energy is high, the reaction will have a faster rate.

Saturday, April 25, 2015
3 questions
1. Recently I learned how to correctly find the pH of a substance and then find the molarity of it.
2. Recently I completed my practice AP test and I need to improve my scores but I at least got a 3 so that's helpful.
3. I need to work on finding x in the Rice equation.
2. Recently I completed my practice AP test and I need to improve my scores but I at least got a 3 so that's helpful.
3. I need to work on finding x in the Rice equation.
Wednesday, April 15, 2015
Calorimeter explore
The difference between an acid and a base in the terms of pH is that acids have more hydrogen ions and bases have more hydroxide ions. The link between pH and pOH is the sum of both equal 14. pH is related to H+ ions because it measures the concentration of H+ ions with 1 being the most acidic to 14 being the most basic. pOH is the measure of the concentration of OH- ions. The universal indicator reacted with every buffer, unlike the other indicators that had limited range of reactions. For a neutralization of 5 or 9, I would choose different indicators considering they are on opposite sides of the spectrum. Indicators are substances that react with either the H+ or OH- ions in another substance, showing a change in color when they react and giving evidence of neutralization. The universal indicator can react with almost any substance, the other indicators have a smaller range of reactivity.





Tuesday, April 14, 2015
3 Questions
1. Recently I've completed my graduation announcement cards. It's coming up so fast!
2. Recently I've learned about letting go of things that aren't in my power. Kind of a hard lesson to grasp.
3. I've been having trouble with some of the math for equilibrium equations, so I'm going to practice those enough that I can do them whilst sleeping.
2. Recently I've learned about letting go of things that aren't in my power. Kind of a hard lesson to grasp.
3. I've been having trouble with some of the math for equilibrium equations, so I'm going to practice those enough that I can do them whilst sleeping.
3 questions
1. Recently I've learned (completely learned) how to finally find molarity after being so confused about it most of the time. It's the small things.
2. Recently I've completed my spring cleaning so that's always refreshing.
3. Right now I'm struggling to find motivation to do anything other than sleep or eat, so I need to work on that. I think I'm just going to try to push myself and finish better than I started.
2. Recently I've completed my spring cleaning so that's always refreshing.
3. Right now I'm struggling to find motivation to do anything other than sleep or eat, so I need to work on that. I think I'm just going to try to push myself and finish better than I started.
Friday, April 10, 2015
Nitrogen Dioxide and Dinitrogen Tetraoxide
The two substances in this reaction are NO2 and N2O4. This reaction is a reversible reaction, which is how it reaches equilibrium.takes more energy. These two are the main components in smog, and the formation of N2O4 reaction The whole reaction is 2NO2 --> N2O4 with an enthalpy of -58.0 kJ/mol of N2O4
The reaction is temperature favored. The cold ice water favors the N2O4, therefore changing the rate of the production of N2O4 and boosting the molarity of it. The heat from the bunsen burner favors the NO2 side of the reaction, making the rate of NO2 faster than the rate of N2O4.
You can tell the cold favors the N2O4 because N2O4 is naturally a clear gas. But as soon as you move the test tube into the boiling water the gas turns a yellowish brown color, showing that the reaction has reversed and the NO2 is more favored because NO2 in gas form is that color. the NO2, when placed in the boiling water, pops. This is due to the greater number in moles. Le Chatelier's principle can be used to predict the effect of the change of conditions in an equilibrium reaction. The equilibrium shift has to do with enthalpy of the reaction. One side if the reaction favors the release of energy, and one favors the absorption if energy. The color change has to do with the equilibrium trying the compensate for the change that the rate of the reaction is kept the same. The reaction at room temperature is a equal mixture of both NO2 and N2O4 color with neither one favored more than the other, compared to the favoring of the reaction side depending on the temperature.
Subscribe to:
Comments (Atom)


